What Is the Best Algae Strain to Eat

Nutrient Ingredients and Nutraceuticals from Microalgae: Chief Product Classes and Biotechnological Production
1
Institute of Biotechnology and Biochemical Engineering, Graz University of Applied science, NAWI Graz, Petersgasse 10-12/I, 8010 Graz, Austria
2
Institute of Biochemistry, Graz University of Technology, NAWI Graz, Petersgasse 10-12/Ii, 8010 Graz, Republic of austria
*
Writer to whom correspondence should be addressed.
Academic Editor: Joana South. Amaral
Received: 8 June 2021 / Revised: nine July 2021 / Accepted: 12 July 2021 / Published: 14 July 2021
Abstract
Microalgal products are an emerging class of nutrient, feed, and nutraceuticals. They include dewatered or dried biomass, isolated pigments, and extracted fat. The oil, protein, and antioxidant-rich microalgal biomass is used as a feed and food supplement formulated equally pastes, powders, tablets, capsules, or flakes designed for daily use. Pigments such as astaxanthin (crimson), lutein (yellow), chlorophyll (green), or phycocyanin (bright blueish) are natural food dyes used as isolated pigments or pigment-rich biomass. Algal fat extracted from certain marine microalgae represents a vegetarian source of n-3-fatty acids (eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), γ-linolenic acid (GLA)). Gaining an overview of the production of microalgal products is a fourth dimension-consuming task. Here, requirements and options of microalgae cultivation are summarized in a concise manner, including lite and nutrient requirements, growth weather condition, and tillage systems. The rentability of microalgal products remains the major obstruction in industrial application. Key challenges are the high costs of commercial-scale cultivation, harvesting (and dewatering), and production quality assurance (toxin analysis). High-value food ingredients are commonly regarded equally profitable despite pregnant capital expenditures and energy inputs. Improvements in capital and operational costs shall enable economic production of low-value nutrient products going downwardly to fishmeal replacement in the future economy.
1. Introduction
Microalgae are microscopic photosynthetic organisms that are found in marine, also as in freshwater, environments. These unicellular organisms have a size ranging from a few to several hundred micrometers, depending on class and species. Most microalgae accept photosynthetic mechanisms similar to land-based plants but generally more efficient in the photoautotrophic conversion of solar free energy into biomass. This is since the cellular construction of microalgae is less sophisticated, and the normal environment is aqueous with easy access to CO2 and farther nutrients [1]. Industrially significant organisms are prokaryotic cyanobacteria, as well as eukaryotic microalgae that include diatoms, golden microalgae, and some greenish microalgae species. Cyanobacteria (Cyanophyceae or blueish-greenish algae) include the well-known Spirulina species Arthrospira platensis and Arthrospira maxima (aka Spirulina platensis and Spirulina maxima). Bluish-green algae are found in a diversity of habitats. Diatoms are a major group of microalgae. Living in the oceans, the diatoms make upward a significant portion of the biomass on World. They often accrue oils and chrysolaminarin (a storage polysaccharide). Light-green algae are peculiarly abundant in freshwater. The principal storage compound of greenish algae is starch, although oils can exist produced as well. The freshwater green algae Haematococcus pluvialis is commercially of import as a source of astaxanthin, Chlorella vulgaris every bit a supplementary food product or food ingredient, and the halophilic algae Dunaliella salina as a source of β-carotene. The golden algae are known for producing oils and carbohydrates [two]. Some prokaryotic and eukaryotic microalgae are known for the production of toxins that can contaminate the environment too as algal cultures [three]. The chemical structures of the toxins contain peptides (e.g., microcystin), alkaloids (e.g., saxitoxin), and alkyl phenols (east.thou., aplysiatoxin).
Microalgae are a resource for food and feed with high potential. Currently, the most cultured species are Arthrospira platensis and Arthrospira maxima, Chlorella vulgaris, Dunaliella salina, and Haematococcus pluvialis, which can be grown photoautotrophically with sunlight as an free energy source. The carbon source is COii, which tin can be supplied by gassing or supplementing the water with soluble carbonates. Heterotrophic cultivation on organic carbon sources such as sugars is used for the production of n-3 fatty acids with marine organisms (Crypthecodinium cohnii, Schizochytrium sp. TC 002, and Ulkenia sp. strain TC 010) in fermenters excluding sunlight [4]. For feed for fish aquaculture, the main cultivated species are: Chlorella vulgaris, Isochrysis galbana, Pavlova sp., Phaeodactylum tricornutum, Chaetoceros sp., Nannochloropsis oceanica, Skeletonema sp., Thalassiosira sp., Haematococcus pluvialis, and Tetraselmis suecica [two,v,6]. However, the technology to produce microalgae is still young. Research and development accept been done in recent years and continue on cultivation systems. A leap in the evolution of microalgae applied science is required; on a practical level, the scale of production needs to increase with a concomitant decrease in the cost of production [1]. Microalgae are cultivated in a wide range of different cultivation systems that can be placed outdoors or indoors. Cultivation systems range from open shallow raceway ponds to closed photobioreactors. The systems mostly used on a big scale and on a commercial basis are open systems [7]. For human consumption, the microalgae Arthrospira platensis and Arthrospira maxima are the most common and well-nigh intensively investigated organism. In nature, these microalgae grow in specific lakes with high pH effectually the earth. As these microorganisms comprise gas, they are floating on the water and tin exist harvested easily from the surface, every bit is done at lake Texcoco (Mexico), Lake Chad, and Lake Kossorom (both in Chad) [1].
The amount of literature roofing microalgae product and employ in foods (and feed) is overwhelming. Therefore, the scope of the nowadays review is to give an overview of microalgae production in a concise manner, including cultivation (growth conditions, cultivation systems, limitations) and downstream processing. Because of its nutritionally excellent composition (amino acids, vitamins, lipids), the microalgae are eaten as is, are included in traditional foods as ingredients, or every bit food additives (colorants).
2. Microalgae Production
A literature search using the keywords "microalgae tillage" results in tens of thousands of hits, with approximately one-half of the manufactures published in the terminal v years. Therefore, and despite dozens of comprehensive review articles, gaining an overview remains a time-consuming task. Hither, nosotros strive to cover the necessary considerations in microalgae cultivation in a concise manner, including illumination and further growth conditions, tillage systems, microbial contaminations, and downstream processing.
two.1. Microalgae Cultivation
2.ane.1. Illumination
Low jail cell densities and moderate growth rates are the major obstacles towards a broad commercial use of microalgal products. The well-nigh important cultivation parameters are lite and nutrient supply. Decisive factors of light supply are low-cal intensity, spectral range, and photoperiod. Optimal calorie-free supply is case-dependent with regard to the type of microalgae simply besides to the type of product. By and large, the charge per unit of photosynthesis correlates with light supply until photo-inhibition is reached. Excessive illumination causes damage to the photoreceptor system leading finally to cell decease [7,8]. Therefore, photosynthetic organisms contain pigments for calorie-free-harvesting and photoprotection. Lite-harvesting pigments such equally chlorophylls, phycobilins, several carotenes, and xanthophylls are structural and functional components of the photosynthetic apparatus. Photoprotective pigments are astaxanthin, β-carotene, and other carotenoids. Production of light-harvesting pigments is supported by subsaturating light conditions (photoacclimation; pigment concentrations typically 0.5 chiliad/gCDW). Photoprotective pigments are overproduced in response to environmental stress, such every bit oversaturating lite intensity, loftier salt concentration, or nitrogen limitation (pigment concentrations up to 70 mg/1000CDW) [9,10].
two.ane.2. Diverse Growth Atmospheric condition
Microalgae show various metabolic pathways. Other than the dominant photoautotrophy, secondary heterotrophy and mixotrophy is encountered (Figure i). All microalgae are photoautotrophs, with autotrophic cultivation being the main method for biomass production [xi]. Photoautotrophs use lite as an energy source to fix carbon via photosynthesis. Big-calibration, photoautotrophic tillage is mostly realized in open systems. Strains that tolerate/require high salinity or alkaline pH are used then that cultures are protected from contaminants. Prominent examples are Arthrospira platensis and Dunaliella salina [12]. Mostly, lower biomass concentrations are obtained with microalgae cultivated photoautotrophically compared to mixotrophic and heterotrophic cultivation strategies [13]. Microalgae capable of mixotrophic growth can metabolize CO2 and light simultaneously with organic carbon sources but can also switch to sole phototrophic or sole heterotrophic growth. These microalgae show college growth rates under mixotrophic atmospheric condition as compared to sole heterotrophic or photoautotrophic conditions [14]. A prominent example of mixotrophic tillage is astaxanthin production by H. pluvialis (e.1000., [15]). Mostly, mixotrophic growth requires less light compared to photoautotrophic growth. Heterotrophs can grow on organic carbon in the absenteeism of light (no illumination required). Again, growth rates under heterotrophic conditions are higher than those under photoautotrophic conditions. Nevertheless, other microorganisms compete with heterotrophic microalgae for the carbon source, and sterile conditions are required to avoid civilization contagion and product loss [12,13].
2.2. Cultivation Systems
Central considerations in the set-up of algae cultivation are the decisions between open ponds (mostly outdoor) or closed photobioreactors (mostly indoor). A uncomplicated characterization of the main cultivation systems is given in Tabular array 1.
2.two.1. Open Ponds
Classical algae cultivation uses open ponds in natural habitats or shallow artificial ponds ofttimes mixed by a rotating arm or by paddle wheels (Effigy 2). Mixed, open ponds, i.due east., raceway-ponds or circulating-open-ponds, are characterized by low power requirements, operating costs, and capital letter costs. However, open-pond cultivation is prone to risks of contamination and other pollution, low possibilities of parameter control (especially illumination), loss of water past evaporation, and high weather dependence. The main limitations stem from bereft mixing, causing a suboptimal supply of CO2 and restricted illumination. Cultivation times are approximately 6–viii weeks, with prison cell densities of about 0.1 or 25 one thousand/mtwod. Furthermore, weather-dependent tillage parameters and unsterile cultivation conditions lead to low reproducibility [seven]. The utilise of controlled, closed photobioreactor systems (PBRs) results in higher biomass (and product concentrations) and reproducible results. A thirteen-fold higher volumetric biomass productivity of PBRs compared to raceway ponds in the cultivation of microalgae for biodiesel production was reported by Chisti [sixteen].
two.2.2. Closed Photobioreactors
PBRs facilitate increased biomass and production concentrations. PBRs protect the algal civilisation against (microbial) contaminations, provide nutrients, illumination, pH and temperature command, and outgassing of the produced oxygen. Mixing and illumination are over again the most important parameters. Optimal cultivation weather condition provide gentle mixing so that cells, carbon dioxide, and other essential nutrients are evenly circulated. Cell movement facilitates constant illumination and prevents prison cell growth on vessel walls (see also Figure 3). The simplest PBRs consist of transparent plastic numberless or plastic columns operated as chimera columns and airlift reactors (also referred to every bit vertical tubular reactors) (Figure 3). The systems are mixed by the bubbles of CO2-enriched air from the bottom. Stirred tank reactor-type (STR) PBRs, such as ones used for bacteria and yeast production, provide the most efficient mixing. Many microalgae strains are, still, sensitive to shear forces. Horizontal tubular reactors (HTRs), made from glass or plastic tubes, accept succeeded within the product calibration [17]. Aeration is accomplished in the tube connections and illumination over the length of the tube [viii,18]. Clearly, microalgae tillage in closed bioreactors is more energy-intensive compared to open ponds [19].
2.three. Contaminations
A major clogging in the industrial production of products from algae is that algae cultures are decumbent to contaminations. To avoid contamination, closed systems tin can exist used; withal, in open systems (large scale), contamination is unavoidable. These are either organisms that graze upon and feed on the algae or undesired algae strains that take over the cultivation media. Contaminations can lead to civilization crashes and product loss, and as well the formation of toxins [xx,21]. Therefore, effective strategies to avert, repress, monitor, and control contaminants without interfering with the growth of the target microalgae are required. Grazers and contaminants experienced (particularly in open up-swimming cultivations) are mainly insect larvae, protozoans (ciliates, amoeba, dinoflagellates), viruses, fungi, bacteria, and other algae strains [22]. Selective growth conditions are effective measures against grazers and contaminants. The strict photoautotrophs Arthrospira platensis and Dunaliella salina, two of the most ofttimes cultivated microalgae, can grow in extreme environments. A. platensis tolerates high bicarbonate concentrations (0.two M) and pH values of up to 10.two, whereas halophilic Dunaliella salina tolerates NaCl concentrations upwardly to four.0 M [23]. Haematococcus pluvialis cultures that are grown under mixotrophic conditions for astaxanthin production are much more susceptible to contaminations. Wen and co-workers [xv] described a successful strategy to cultivate Haematococcus pluvialis under mixotrophic weather in an outdoor raceway pond. Haematococcus pluvialis cultivation started under photoautotrophic conditions until nitrate is depleted from the medium, and then the culture is supplemented with acetate/acetic acrid. Under mixotrophic conditions, Haematococcus pluvialis cells grow intracellular nitrogen pool, while bacterial reproduction is express. Further measures against grazers and contaminants include the addition of chemicals (hypochlorite, pH shocks, ozone), add-on of pesticides, or herbicides [15,24,25]. Some other approach is the co-cultivation of microalgae to obtain a more robust polyculture compared to monocultures [26].
2.four. Downstream Processing
Similar to the bulk of bioprocesses, downstream processing constitutes a considerable cost gene. Specifically, the toll for the harvest of microalgae was calculated as 20–xxx% of the total product toll of cheap majority products such equally biodiesel [27]. The primary bug in microalgae separation are small cell sizes (2 to 30 µm), wearisome gravity settling (<ten−5 1000 southward−i), and depression cell densities [28]. Harvesting requires one or more than liquid-solid separation steps such as coagulation, flocculation, sedimentation, flotation, filtration, and centrifugation. Nearly microalgae cells exercise non aggregate spontaneously (an exception is Anabaena sp. ATCC 33047) [29]. Cell-jail cell contact is prevented by repulsion due to negatively charged jail cell surfaces. A change of pH or electrolyte addition reduces surface charges, facilitate close prison cell-cell contact resulting in van der Waals attraction and coagulation. Similarly, cationic polymers screen negative charges, span cells, and lead to flocculation. Gravity settling (sedimentation) depends on cell bore and density differences between cells and medium. Settling velocities of unmarried cells are low (<x−5 1000 due south−one). Therefore, coagulation, flocculation, and flotation are frequently used to advance sedimentation (~10−4 m s−ane). During flotation, ascent air bubbles (10 to 100 µm) attach to cells and aid in collecting cells at the liquid surface [28,xxx]. Flocculated cells can be separated past macrofiltration membranes (pore sizes >10 µm) with the advantage of a lower energy requirement (i.e., lower pressure). In a previous study, crossflow ultrafiltration and microfiltration for the concentration of Dunaliella salina cells were compared. Frequent backflushing to alleviate membrane fouling (intrapore fouling and block germination/pore-blocking) was required to maintain the transmembrane flow. Interestingly, average permeate fluxes and cell integrity losses were similar in the two systems. Rapid drops in transmembrane fluxes with the microfiltration membrane were ascribed to severe intrapore fouling. A clearer permeate was obtained with ultrafiltration [31]. Microalgae harvesting past centrifugation requires high capital investment and energy costs and is frequently used in the manufacturing of loftier-value products. Economic considerations crave preconcentration steps in the industrial production of medium and depression-cost products.
In the majority of applications, subsequent energy-intensive dewatering and biomass drying steps are required. Give-and-take of farther downstream processing is highly strain and product-specific. Extraction of oils and dyes requires prison cell rupture, centrifugation, and/or solvent extraction [31]. The conditions of tillage, harvesting, and subsequent processing conspicuously influence the nutritional limerick of microalgae. For example, protein loss past the drying of Arthrospira platensis was previously reported. With freeze-drying, 5% of protein was lost, whereas spray, convective, and infrared drying led to protein losses of 10 upwardly to 25% [32].
3. Rentability of Microalgae Production
One of the major concerns relates to the rentability of microalgal products. Key challenges are the loftier production costs of products made up of commercial-scale cultivation, harvesting (dewatering), and finally, product quality assurance. The value of microalgal products spans from uncommonly high market values (e.g., EPA 4600 USD/kg) to products in the middle toll segment (due east.m., biomass for food and feed 10 to l USD/kg) down to fishmeal replacement (fishmeal cost is approximately 2 USD/kg) [33]. Several algal-based foods have been launched in the terminal years: Microalgae biomass is used in the form of powders, tablets, and capsules. Especially in Western countries, the number of snacks and drinks containing microalgae doubled in the past few years. Virtually new products launched were baker, meals, and chocolate confectionery [34]. Improvements in upper-case letter and operational costs shall enable economical product of low-value food and feed products going down to fishmeal replacement in the future economy [33,35].
iv. Nutritional Composition of Microalgae
The composition of the microalgae is like to other bacteria. Usually, they contain high amounts of protein, with all essential amino acids present. Especially the microalgal species Arthrospira platensis, Chlorella vulgaris, Dunaliella salina, Haematococcus pluvialis, and Scenedesmus obliquus show a very high content of poly peptide with values higher than soybean, corn, and wheat (Table 2). Polyunsaturated fatty acids are occurring in significant amounts, including linoleic acrid, γ-linolenic acid (GLA), eicosapentaenoic acid (EPA), docosahexaenoic acrid (DHA), and arachidonic acid (Tabular array 3). Mostly, microalgae show a high protein content when cultured in media with high concentrations of nitrogen source and nether well-counterbalanced growth conditions. The accumulation of lipids and carbohydrates is induced under stress atmospheric condition. (Note that green algae show a nitrogen requirement that corresponds to 5–10% of their biomass. Culture media adult for microalgae cultivation contain between five and 50 mM to avert nitrogen limitation) [36]. In addition, a series of h2o-soluble vitamins are present. Bioactive vitamin B12 (cobalamin) is normally not present in microalgae as it is synthesized equally pseudocobalamin in planktonic cyanobacteria [37]. A series of minerals, including iodine, is also occurring [38]. Although iodine can occur in college concentrations, it rarely poses a health problem, interim mainly by interfering with the thyroid gland metabolism resulting in thyrotoxicosis [39,40]. A serial of pigments are as well available that are involved in photosynthesis. These comprise chlorophyll a, a series of carotenoids (e.thou., β-carotene, zeaxanthin, β-cryptoxanthin) and, in Arthrospira platensis, the blue pigments c-phycocyanin and allo-phycocyanin [41]. Other than being a valuable source of macronutrients, the microalgae can also contain polyunsaturated fatty acids. Of special interest are the essential fatty acids comprising linoleic acid and the due north-three fatty acids. Dolganyuk and co-workers studied the lipid complex of dissimilar microalgae (Chlorella vulgaris, Dunaliella salina, Arthrospira platensis). They plant a huge variation in the fatty acrid composition, especially in the due north-3 to north-6 ratio, with Dunaliella salina containing the highest amount of n-iii fatty acids (ALA, DHA) [2].
The analyses of the protein composition have shown that, for most of the microalgal species (except Aphanizomenon sp.), the eight essential amino acids occur in equal or higher amounts than recommended by the WHO/FAO [44]. The quality of the proteins with respect to biological value, digestibility, utilization, and poly peptide efficiency ratio is equal to conventional establish proteins [45].
Sandgruber and co-workers published the compositional analyses of a series of commercially available dried microalgal products [46]. The concentrations of the minerals (Ca, Mg) and the trace elements (Fe, Mn, Ni, Cu, Zn, Mo, Se, I) showed a high variation between the species. Even when analyzing different products of the aforementioned species, a high variation was observed. This is particularly the case with calcium, magnesium, and zinc having a variation of two to three orders of magnitude. A similar situation was observed with the heavy metals Every bit, Cd, Hg, and Lead.
5. Food Ingredients and Nutraceuticals from Microalgae
5.1. Microalgae Used as a Food Ingredient
When using microalgae as a food ingredient, some limitations are obvious. This is primarily because of the intensive light-green color that is added to the recipes and a fishy aroma [47]. As the flavor is one of the biggest disadvantages of algae-containing nutrient, a lot of work was invested in masking this flavor past using intensive spices [48] or by using the olfactory property of the microalgae to bring the typical smell of seafood out. A commercial product [49,50] was developed that shows microalgae can be used as an ingredient in foods with a typical seafood odor. Many products from algae are commercialized directly as biomass. These are sold equally nutritional supplements since the microalgae, mostly Arthrospira platensis, incorporate loftier amounts of protein and n-3 fatty acids. Other products derived from Haematococcus pluvialis are used for supplementing the carotenoids astaxanthin and lutein. Since microalgae are also known for having loftier concentrations of due north-3 fatty acids (mainly Chlorella vulgaris and Arthrospira platensis containing γ-linolenic acid, eicosapentaenoic acid, docosapentaenoic acid, docosahexaenoic acid, Table 2), extracts are developed that might be used to upgrade foods with these fatty acids.
five.i.one. Addition of Microalgae to Biscuits
Spray-dried biomass of Arthrospira maxima tin can be used as an ingredient for biscuits that tin can improve the nutritional quality [48]. In their experiments, da Silva and co-workers could testify that the content of iron was improved in parallel to the protein and lipid content. In add-on, the products were enriched with valuable amino acids and polyunsaturated fatty acids with high amounts of γ-linolenic acid (n-six fatty acid). It is interesting to note that the sensory profile of the biscuits was acceptable to the consumers with a limitation related to the color. Up to an addition of xx% of spray-dried biomass, the sensory score was acceptable. In this case, the Arthrospira maxima biomass was spray-dried in the presence of starch sodium octenyl succinate (East 1450, emulsifier, foaming agent). The skilful sensory properties of the last biscuits were attributed to the presence of the nutrient additive, which might mask the typical algal aroma. The improver of microalgal biomass to modify the texture of foods is some other possibility to include the cyanobacteria [51]. The idea behind the concept of Bernaerts and co-workers was to apply the biopolymers that are occurring in microalgae to modify the viscosity of the nutrient product into which the microorganisms are integrated. These polymers incorporate the intracellular proteins and starch-like structures that are liberated past the disintegration of the cells besides as the prison cell wall polysaccharides and exopolysaccharides. However, the variety of the polysaccharides is huge, and it is hard to predict the composition and relate it to the chemical backdrop. Due to this diversity of structures and compositions, there is limited knowledge of the rheological properties and potential applications for foods [42]. In add-on to these biopolymers, resistant polymers tin can be present in some species (e.g., Haematococcus pluvialis, Chlorella sp., Scenedesmus sp., Nannochloropsis sp.). These are called algaenans, existence polymers of carbon chains that are cross-linked by ether and ester bonds. Sahni and co-workers [52] investigated if the remaining biomass afterward extraction of chlorophyll could be used as an ingredient in the training of cookies. They studied how much of the wheat flour could be replaced by the microalgal repast that originated from Chlorella sp. (Abca-17). Some of the textural parameters inverse with the addition of 1–12% of the microalgal meal, i.e., the weight and thickness increased, whereas the diameter, spread ratio, and spread factor decreased with increasing content. Past adding up to 6% microalgal powder, no significant difference was found in a sensory evaluation. A change in the mouthfeel was besides observed, meaning that the necessary force for breaking the fortified cookies increased in parallel with the improver. The observed colour changes were a concealment and increased greenness in the cookies with increasing content of the algal biomass. The improver of Arthrospira platensis powder to a standard recipe for cookies with concentrations of up to iii% resulted in an increase of hardness and lower sensory scores. The color alter was obvious even at the lowest tested level of algae addition of 1% [53].
5.1.2. Addition of Microalgae to Pasta and Noodles
Flours obtained from Chlorella vulgaris and Arthrospira platensis in combination with the macroalgae Eucheuma cottonii were added to wheat flour to produce noodles. Information technology was shown that when adding the algal biomass to the dough of the noodle, the content of protein, fat, ash, and dietary fiber increased, whereas the carbohydrate content reduced with no changes in moisture. These changes are due to a new composition of the ingredients. The therewith produced noodles were shown to have acceptable culinary properties, such as texture, color, olfactory property, and flavour. As an example, the optimal composition of the flour consisted of 90 g wheat flour, 5 g Arthrospira platensis, and 5 yard Eucheuma cottonii dried biomass resulting in noodles with a depression fat and loftier poly peptide content [54]. Since pasta is a staple food consumed worldwide, information technology might be an excellent target for improving the nutritional status past calculation high-value ingredients. When developing recipes with new ingredients that amend the nutritional value, it has to be considered that not only the texture and cooking properties might modify only also the sensory properties. De Marco and co-workers [55] could show that up to 20 1000 of Arthrospira platensis pulverization could exist added per 100 1000 of flour with a result of a technologically adequate product. Similar results were obtained by the group of El-Baz [56], who investigated the improver of a powder of the microalgae Dunaliella salina to pasta preparation. The sensory evaluation of the pasta was changing with a microalgae content of more than two%. Up to 2%, the mouthfeel and overall acceptability was non changed. The color changed to greenish, which is a outcome of the chlorophyll being introduced with the microalgae. A comparable experiment was carried out using Chlorella vulgaris and Arthrospira maxima equally an ingredient for pasta. The addition of algae was up to 2% resulting in products with different colors. Greenish (chlorophyll) or orangish (cantaxanthin) for pasta with added chlorella and green with a blue shade (probably because of the presence of blueish phycocyanin) for pasta with Arthrospira platensis. The texture improved with increasing amounts of integrated algal biomass, which might have been due to the higher content of protein and lower h2o uptake. However, the products with higher contents of algae had a lower sensory score [57].
5.2. Isolated Products from Microalgae for Enrichment of Foods
five.2.1. n-3 Fatty Acids
Some of the cyanobacteria are known for their high content of north-3 fat acids (Table iii). In add-on to these, a series of species are known for containing EPA and DHA in the range of 17–45% of the full lipids (e.m., Nannochloropsis oceanica and Nannochloropsis salina, Pinguiococcus pyrenoidosus, Thraustochytrium sp., Chlorella minutissima, Dunaliella salina, Pavlova viridis and Pavlova lutheri, Isochrysis galbana, Schizochytrium sp., Crypthecodinium cohnii, Aurantiochytrium sp., Phaeodactylum tricornutum) [58]. Since the alimentary supply of due north-3 fat acids is generally limited due to express availability of fish oils, a series of efforts are fabricated to increase the content of these fatty acids in foods. One of the possibilities is to enrich food products with blue-green alga containing high amounts of highly unsaturated fatty acids [59]. Adding microalgae to pasta, in full general, can improve the nutrient composition, as discussed before. The chief problem associated with highly unsaturated fat acids is their low oxidative stability, since deteriorated products produce a rancid aroma in addition to the health problems related to the uptake of oxidized oils [60]. Notwithstanding, from a nutritional point of view, the uptake of due north-3 fat acids from microalgal oil is comparable to fish oil with the possible advantage of a better supply with carotenoids [61]. In add-on to adding the n-3 fat acids directly to the food, it is possible to enrich animate being products by supplying the unsaturated fatty acids with the feed. This was shown, for case, past Lemahieu and a co-worker [62], who could increase the DHA content of eggs when feeding Isochrysis galbana to the chicken. Polyunsaturated fatty acids (PUFAs), particularly the n-3 fatty acids having five (EPA) or six (DHA double bonds, are prone to oxidation due to the lability of the hydrogens linked to the methylene groups between the double bonds. These PUFAs require stabilization against oxidation. Shen and co-workers could show that a combination of octyl gallate with tea polyphenol palmitate showed the virtually constructive protection [63].
5.2.2. Carotene and Other Carotenoids
Microalgae are known for producing high amounts of carotenoids. Dunaliella salina tin can produce β-carotene up to 13% of dry out weight. Astaxanthin can be found in Haematococcus pluvialis, which produces up to 7% of the dry weight. Canthaxanthin was constitute in Coalstrella striolata containing shut to 5% of dry weight. Lutein, zeaxanthin, fucoxanthin, echinenone, and violaxanthin could also be identified in different species [64]. The EFSA Panel on Nutrition, Novel Foods, and Food Allergens concluded that an intake of 8 mg of astaxanthin from food supplements is safe for adults. This is also true for the combination with the background diet [65]. The evaluation of mixed carotenes and β-carotene of different sources showed that no adequate daily intake (ADI) could be established for use as a food colour. However, the total uptake of the carotenoids should be in the range of 5–ten mg/day. This includes the safe exposure to β-carotene of beneath 15 mg/twenty-four hour period, which does not increase the cancer take a chance [66]. A long list of bioactivities of the algal carotenoids that are related to human health was established [67]. In addition to the well-described vitamin A activity and reduction of historic period-related macular degradation (AMD) by lutein [68], these compounds can have an impact on human wellness, comprising anti-cancer, anti-obesity, anti-inflammatory, antidiabetic, antimicrobial, and neuroprotective activities.
five.three. Microalgae as Nutrient Supplements
As mentioned before, the nutritional composition of microalgae is of high quality with respect to amino acids, fatty acids, and pocket-size components such as vitamins and pigments. With these high concentrations of nutrients, microalgae are predestined for the manufacturing of food supplements. The principal microalgae that are marketed as dried powder, capsules, or pressed pills are Chlorella vulgaris and Arthrospira platensis. A series of positive health effects of microalgae are described ranging from an influence on the cholesterol level and associated coronary middle diseases, antiviral, antimicrobial, and antifungal action. The presence of sterols (eastward.g., clionasterol) implies all the potential positive wellness effects that are related to an intake of these compounds [69]. Arthrospira platensis is particularly known for its high content of γ-linolenic acid, 1 of the essential fat acids. It is also rich in water-soluble vitamins. In addition to the effects of Arthrospira platensis, Chlorella vulgaris has been characterized by the presence of dietary antioxidants such equally lutein, α- and β-carotene, and vitamins C and E. In C. vulgaris the most interesting chemical compound is β-1,3-glucan, which might stimulate the human being immune organisation [70]. Astaxanthin, another carotenoid with beneficial health effects, is found in loftier concentrations in Haematococcus pluvialis [71]. Information technology was approved as a food supplement by the EFSA in 2020 [65].
v.4. Toxicological Relevance of Microalgal Products
Some of the cyanobacteria species are known for their potential of producing potent toxins. When these microalgae contaminate the cultures for food production, the toxins pose a severe health risk. Some examples are, microcystin and related structures are produced by Microcystis aeruginosa, saxitoxin by Aphanizomenon flos-aquae, and anatoxins past Anabaena flos-aquae. The chemic structures comprise cyclic peptides (microcystin), alkaloids (anatoxin, saxitoxin), and lipopolysaccharides affecting the liver, nerve axons, and synapse, as well as the alimentary canal [3]. The grouping of Dietrich published an overview on the presence of algal toxins in commercially available food supplements. In their study, they only found microcystins, which were present in products of Aphanizomenon flos-aquae. Extracts from all the tested samples showed cytotoxic effects, which leads to the decision that additional components should be nowadays that have the potential to induce adverse effects in consumers [72].
6. Conclusions
Past. Microalgae have been consumed as a nutrient for a long time: Spanish conquerors reported that Aztecs consumed cyanobacteria Arthrospira platensis and Arthrospira maxima equally a dry cake. In Chad, Arthrospira platensis has been used as food on a daily basis for centuries. The filamentous cyanobacteria Nostoc commune, Nostoc flagelliforme, and Nostoc punctiforme are traditionally consumed in China, Mongolia, Tartaria, and South America. In Japan, the cyanobacterium Aphanotheca sacrum is a traditional effeminateness (reviewed in [73]).
Present. Microalgae-based products for food (and feed) contain either whole dried biomass (Arthrospira platensis, Chlorella vulgaris) or isolated components (astaxanthin, β-carotene, phycocyanin, and the n-3 fatty acids EPA and DHA).
Recently a comprehensive list of commercially available products from microalgae and products containing microalgae as an ingredient was published by Eltanahy and Torky [74].
The steadily increasing involvement in the apply of microalgae in the food industry is based on 2 major developments:
-
New aseptic cultivation systems for the production of pure cultures under controlled conditions to achieve high cell densities. High product concentrations (i.eastward., biomass, fat, secondary metabolites) are a prerequisite for industrial production. Improved cultivation systems facilitate the industrial product of selected species (Arthrospira platensis, Chlorella vulgaris) with high nutritional values (high protein contents) and interesting metabolites (nutrient colorants, antioxidants etc.).
-
Consumers need vegan foods, natural foods, and sustainable food production. Microalgae has go a 'way nutraceutical' in Western countries.
Time to come. Microalgal biomass is considered by scientists, engineers, and investors, along with insects and mycoprotein, as a future nutrient to mitigate malnutrition. Future foods are characterized past high protein content, depression requirement for chemicals (fertilizers, hormones etc.), and a low greenhouse gas footprint [one].
The authors expect that in the near future, (i) the production cost of microalgae will subtract, (ii) microalgae volition exist cultivated for the sake of protein production, and (three) a series of newly developed products from microalgae will enter the food market place.
Author Contributions
Conceptualization, M.M.; Writing—Original Draft Training, M.M. and R.K.; Writing—Review and Editing, Grand.G. and R.K.; Visualization, M.Thou. and R.K. All authors take read and agreed to the published version of the manuscript.
Funding
This enquiry received no external funding.
Acknowledgments
We would similar to thank Claudia Hrastnik for the maintenance and cultivation of Arthrospira platensis and Open Access Funding past the Graz Academy of Applied science.
Conflicts of Involvement
The authors declare no conflict of interest.
References
- Habib, One thousand.A.B.; Parvin, Yard.; Huntington, T.C.; Hasan, Yard.R. A Review on Culture, Production and Utilise of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; FAO Food and Agronomics Organisation of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Dolganyuk, V.; Andreeva, A.; Budenkova, E.; Sukhikh, S.; Babich, O.; Ivanova, S.; Prosekov, A.; Ulrikh, E. Study of morphological features and decision of the fatty acrid composition of the microalgae lipid complex. Biomolecules 2020, 10, 1571. [Google Scholar] [CrossRef] [PubMed]
- Katircioglu, H.; Akin, B.S.; Atici, T. Microalgal toxin(s): Characteristics and importance. Afr. J. Biotechnol. 2004, 3, 667–674. [Google Scholar]
- Karnaouri, A.; Chalima, A.; Kalogiannis, Grand.G.; Varamogianni-Mamatsi, D.; Lappas, A.; Topakas, E. Utilization of lignocellulosic biomass towards the production of omega-3 fatty acids by the heterotrophic marine microalga Crypthecodinium cohnii. Biores. Technol. 2020, 303, 122899. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, Chiliad.; Gislerød, H.R. Fatty acid limerick of 12 microalgae for possible utilize in aquaculture feed. Aquacult. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Bhattacharjya, R.; Marella, T.K.; Tiwari, A.; Saxena, A.; Singh, P.M.; Mishra, B. Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Biores. Technol. 2020, 318, 24073. [Google Scholar] [CrossRef] [PubMed]
- Koller, G. Design of closed photobioreactors for algal cultivation. In Algal Biorefineries; Prokop, A., Bajpai, R.Thou., Zappi, One thousand.E., Eds.; Springer: Heidelberg, Frg, 2015; pp. 133–186. [Google Scholar]
- Huang, Q.; Jiang, F.; Wang, 50.; Yang, C. Blueprint of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial involvement. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.-Due south.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment product: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Daneshvar, Due east.; Ok, Y.Due south.; Tavakoli, South.; Sarkar, B.; Shaheen, S.K.; Hong, H.; Luo, Y.; Rinklebe, J.; Vocal, H.; Bhatnagar, A. Insight into upstream processing of microalgae: A review. Biores. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Wen, Ten.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2020, 28, 625–638. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Guerra, I.; Pereira, H.; Costa, M.; Silva, J.T.; Santos, T.; Varela, J.; Mateus, 1000.; Silva, J. Operation regimes: A comparison based on Nannochloropsis oceanica biomass and lipid productivity. Energies 2021, xiv, 1542. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed organisation designs and performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.J.; Harrison, S.T.50. Aeration energy requirements for lipid production by Scenedesmus sp. in airlift bioreactors. Algal Res. 2014, five, 249–257. [Google Scholar] [CrossRef]
- Ger, 1000.A.; Urrutia-Corderob, P.; Frost, P.C.; Hansson, 50.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Jang, K.-H.; Ha, K.; Joo, Thou.-J.; Takamura, N. Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshw. Biol. 2003, 48, 1540–1550. [Google Scholar] [CrossRef]
- Karuppasamy, Due south.; Musale, A.S.; Soni, S.; Bhadra, B.; Gujarathi, North.; Sundaram, M.; Sapre, A.; Dasgupta, South.; Kumar, C. Integrated grazer management mediated by chemicals for sustainable cultivation of algae in open ponds. Algal Res. 2018, 35, 39–448. [Google Scholar] [CrossRef]
- Richmond, G. Biological principles of mass cultivation of photoautotrophic microalgae. In Handbook of Microalgal Civilisation, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley: Chichester, U.k., 2013; pp. 169–204. [Google Scholar]
- Koller, Thou.; Salerno, A.; Tuffner, P.; Koinigg, Thousand.; Böchzelt, H.; Schober, S.; Pieber, Due south.; Schnitzer, H.; Mittelbach, M.; Braunegg, G. Characteristics and potential of micro algal cultivation strategies: A review. J. Clean Prod. 2012, 37, 377–388. [Google Scholar] [CrossRef]
- Day, J.; Thomas, J.; Achilles-Mean solar day, U.E.; Leakey, R.J. Early detection of protozoan grazers in algal biofuel cultures. Biores. Technol. 2012, 114, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Tejido-Nuñez, Y.; Aymerich, Eastward.; Sancho, L.; Refardt, D. Co-cultivation of microalgae in aquaculture water: Interactions, growth and food removal efficiency at laboratory- and airplane pilot-scale. Algal Res. 2020, 49, 101940. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.50.; Simões, M.; Pires, J.C.Chiliad. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Grima, E.Thou.; Fernández, F.G.A.; Robles-Medina, A. Downstream processing of cell mass and products. In Handbook of Microalgal Civilisation, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley: Chichester, Great britain, 2013; pp. 267–309. [Google Scholar]
- González López, C.Five.; Acién Fernández, F.Chiliad.; Fernández Sevilla, J.Thou.; Sánchez Fernández, J.F.; Cerón García, Grand.C.; Molina Grima, E. Utilization of the cyanobacteria Anabaena sp. ATCC 33047 in CO2 removal processes. Biores. Technol. 2009, 100, 5904–5910. [Google Scholar] [CrossRef]
- Koller, 1000.; Muhr, A.; Braunegg, M. Microalgae every bit versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Monte, J.; Sá, Yard.; Galinha, C.F.; Costa, L.; Hoekstrad, H.; Brazinha, C.; Crespo, J.G. Harvesting of Dunaliella salina past membrane filtration at pilot scale. Sep. Purif. Technol. 2018, 190, 252–260. [Google Scholar] [CrossRef]
- Desmorieux, H.; Hernandez, F. Biochemical and physical criteria of Spirulina after different drying processes. In Proceedings of the 14th International Drying Symposium (IDS), São Paulo Urban center, Brazil, 22–25 August 2004; pp. 900–907. [Google Scholar]
- Walsh, Yard.J.; Van Doren, Fifty.K.; Shete, N.; Prakash, A.; Salim, U. Financial tradeoffs of energy and food uses of algal biomass under stochastic conditions. Appl. Energy 2018, 210, 591–603. [Google Scholar] [CrossRef]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.Thou.; Machado Silva, A.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market place innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, 1000.; Barbosa, Yard.; Sijtsma, Fifty. Microalgae-based products for the food and feed sector: An outlook for Europe. In EUR—Scientific and Technical Inquiry Series; Vigani, M., Parisi, C., Cerezo, E.R., Eds.; European Committee, EUR 26255—Joint Enquiry Middle—Institute for Prospective Technological Studies, Publications Office: Luxembourg, 2014. [Google Scholar]
- Amorim, Thou.L.; Soares, J.; dos Reis Coimbra, J.S.; de Oliveira Leite, M.; Teixeira Albino, L.F.; Arêdes Martins, Thousand. Microalgae proteins: Production, separation, isolation, quantification, and application in nutrient and feed. Crit. Rev. Nutrient Sci. Nutr. 2020, 61, 1976–2002. [Google Scholar] [CrossRef]
- Grossman, A. Food conquering: The generation of bioactive vitamin B12 by microalgae. Curr. Biol. 2016, 26, R319–R337. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Clarke, W.; Pratt, South. Cycling of iodine by microalgae: Iodine uptake and release past a microalgae biofilm in a groundwater holding pond. Ecol. Engin. 2016, 94, 286–294. [Google Scholar] [CrossRef]
- Iwamoto, K.; Shiraiwa, Y. Characterization of intracellular iodine accumulation by iodine-tolerant microalgae. Procedia Environm. Sci. 2012, 15, 34–42. [Google Scholar] [CrossRef]
- Müssig, One thousand. Chapter 93—Iodine-induced toxic effects due to seaweed consumption. In Comprehensive Handbook of Iodine; Preedy, Five.R., Burrow, Thousand.N., Watson, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 897–908. [Google Scholar]
- Begum, H.; Yusoff, F.K.D.; Banerjee, Southward.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Nutrient Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Biores. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Lee, T.Fifty.; Gonzalez-Marino, Thousand.E. Microalgae for "healthy" foods—Possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010, nine, 655–675. [Google Scholar] [CrossRef]
- FAO/WHO. Energy and protein requirement. In Report of a Joint FAO/WHO Advertizement Hoc Proficient Committee; FAO: Geneva, Switzerland, 1973; Book 52. [Google Scholar]
- Becker, E.Due west. Micro-algae every bit a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Sandgruber, F.; Gielsdorf, A.; Baur, A.C.; Schenz, B.; Müller, S.M.; Schwerdtle, T.; Stangl, Grand.I.; Griehl, C.; Lorkowski, S.; Dawczynski, C. Variability in macro- and micronutrients of 15 commercially available microalgae powders. Mar. Drugs 2021, 19, 310. [Google Scholar] [CrossRef]
- Lafarga, T. Consequence of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- da Silva, South.P.; Ferreira do Valle, A.; Perrone, D. Microencapsulated Spirulina maxima biomass as an ingredient for the production of nutritionally enriched and sensorially well-accepted vegan biscuits. LWT Food Sci. Technol. 2021, 142, 110997. [Google Scholar] [CrossRef]
- Mantecón, L.; Moyano, R.; Cameánc, A.M.; Jos, A. Safety assessment of a lyophilized biomass of Tetraselmis chuii (TetraSOD®) in a 90 twenty-four hour period feeding study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef]
- Plankton Marino. Available online: https://www.planctonmarino.com (accessed on 28 May 2021).
- Bernaerts, T.G.M.; Gheysen, L.; Foubert, I.; Hendrickx, Thousand.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef] [PubMed]
- Sahni, P.; Sharma, S.; Singh, B. Evaluation and quality assessment of defatted microalgae repast of Chlorella every bit an culling food ingredient in cookies. Nutr. Nutrient Sci. 2019, 49, 221–231. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Zbikowska, A.; Majewska, B. Consequence of Spirulina (Spirulina platensis) improver on textural and quality backdrop of cookies. Ital. J. Food Sci. 2017, 30, i–12. (In Italian) [Google Scholar]
- Kumoro, A.C.; Johnny, D.; Alfilovita, D. Incorporation of microalgae and seaweed in instant fried wheat noodles manufacturing: Nutrition and culinary properties study. Int. Nutrient Res. J. 2016, 23, 715–722. [Google Scholar]
- de Marco, E.R.; Steffolani, Yard.E.; Martínez, C.S.; León, C.South. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- El-Baz, F.Chiliad.; Abdo, S.M.; Hussein, A.M.S. Microalgae Dunaliella salina for apply as food supplement to improve pasta quality. Int. J. Pharm. Sci. Rev. Res. 2017, 46, 45–51. [Google Scholar]
- Fradique, Grand.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, Northward.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, Thou.C.; Gouveia, L.; Bandarra, Due north.K.; Raymundo, A. Isochrysis galbana and Diacronema vlkianum biomass incorporation in pasta products as PUFA's source. LWT Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef]
- Böhm, T.; Berger, H.; Nejabat, M.; Riegler, T.; Kellner, F.; Kuttke, M.; Sagmeister, S.; Bazanella, M.; Stolze, K.; Daryabeigi, A.; et al. Food-derived peroxidized fatty acids may trigger hepatic inflammation: A novel hypothesis to explain steatohepatitis. J. Hepatol. 2013, 59, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, Yard.; Muylaert, Grand.; Foubert, I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fat acids every bit an culling for fish oil. Food Chem. 2014, 160, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Lemahieu, C.; Bruneel, C.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of different omega-3 polyunsaturated fatty acid (n-3 PUFA) sources (flaxseed, Isochrysis galbana, fish oil and DHA Gold) on n-iii LC-PUFA enrichment (efficiency) in the egg yolk. J. Funct. Foods 2015, 19, 821–827. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, C.; Lu, T.; Ding, X.-Y.; Zhao, M.-T.; Zhang, M.; Liu, H.-L.; Vocal, L.; Zhou, D.-Y. Effects of gallic acrid alkyl esters and their combinations with other antioxidants on oxidative stability of DHA algae oil. Nutrient Res. Int. 2021, 143, 110280. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, 1000.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, xiii, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Console on Nutrition, Novel Foods and Food Allergens); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, 1000.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientifc Opinion on the safety of astaxanthin for its use equally a novel food in food supplements. EFSA J. 2020, 18, 5993. [Google Scholar]
- EFSA NDA Panel (EFSA Console on Nutrition, Novel Foods and Nutrient Allergens); Turck, D.; EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific stance on the re-evaluation of mixed carotenes (E 160a(i)) and beta-carotene (E 160a (ii)) equally a food additive. EFSA J. 2012, 10, 2593. [Google Scholar]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, Thou.A. Xanthophylls from the sea: Algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Fen, L.; Nie, Chiliad.; Jiang, H.; Fan, W. Furnishings of lutein supplementation in age-related macular degeneration. PLoS ONE 2019, 14, e0227048. [Google Scholar]
- Plaza, M.; Cifuentes, A.; Ibanez, E. In the search of new functional food ingredients from algae. Trends Nutrient Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Vonshak, A. Astaxanthin aggregating in the green alga Haematococcus pluvialis. Plant Jail cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Heussner, A.H.; Mazija, Fifty.; Fastner, J.; Dietrich, D.R. Toxin content and cytotoxicity of algal dietary supplements. Toxicol. Appl. Pharmacol. 2012, 265, 263–271. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; de Vicente, K.; Galán, B. Microalgae, onetime sustainable food and way nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Eltanahy, E.; Torky, A. Microalgae equally jail cell factories: Food and feed-grade high-value metabolites. In Microalgal Biotechnology: Contempo Advances, Market place Potential, and Sustainability; Royal Order of Chemistry: Cambridge, UK, 2021; pp. i–35. [Google Scholar]
Figure one. Free energy and carbon sources of microalgae.
Figure one. Energy and carbon sources of microalgae.
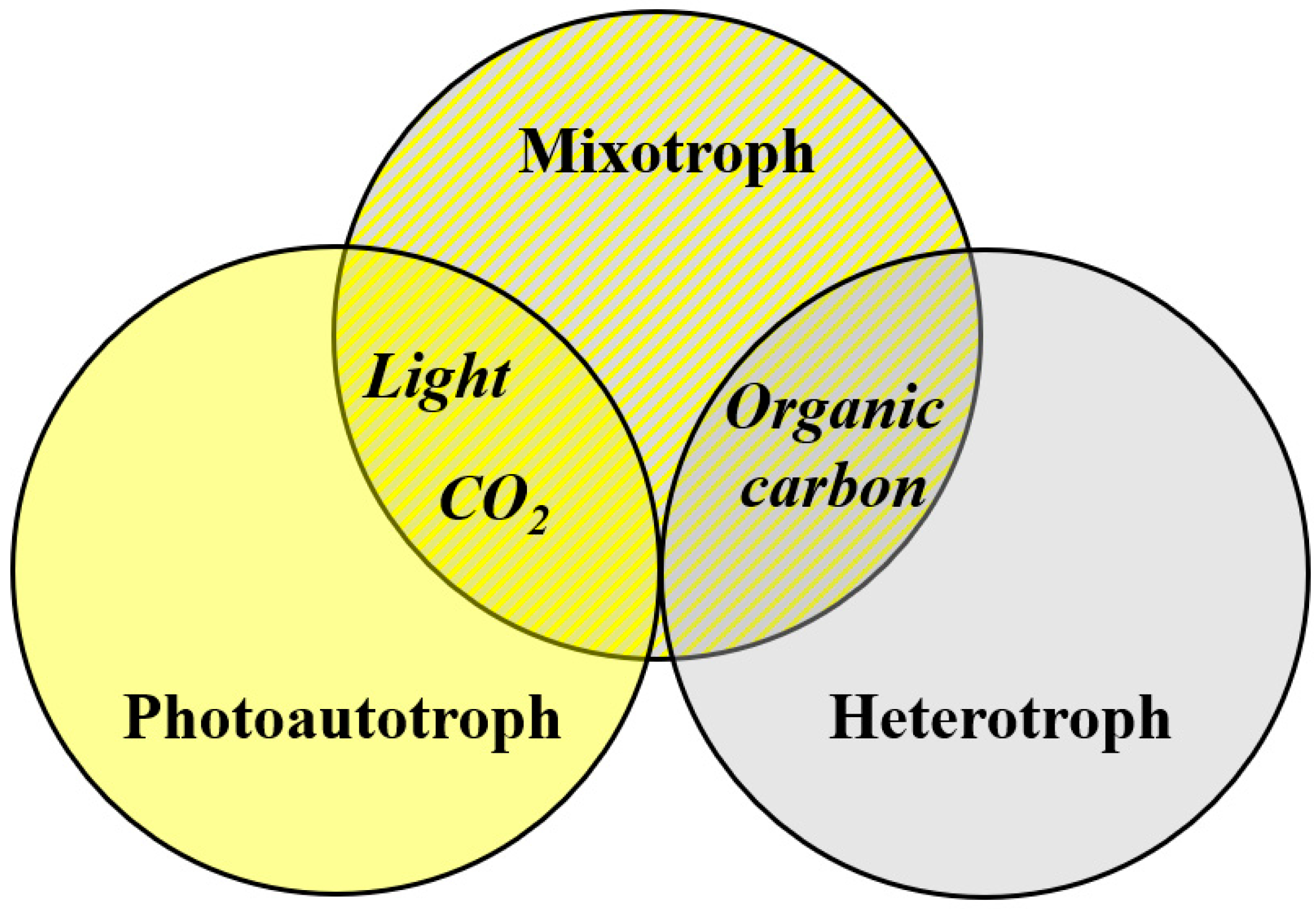
Figure ii. Scheme of a raceway pond.
Figure two. Scheme of a raceway pond.
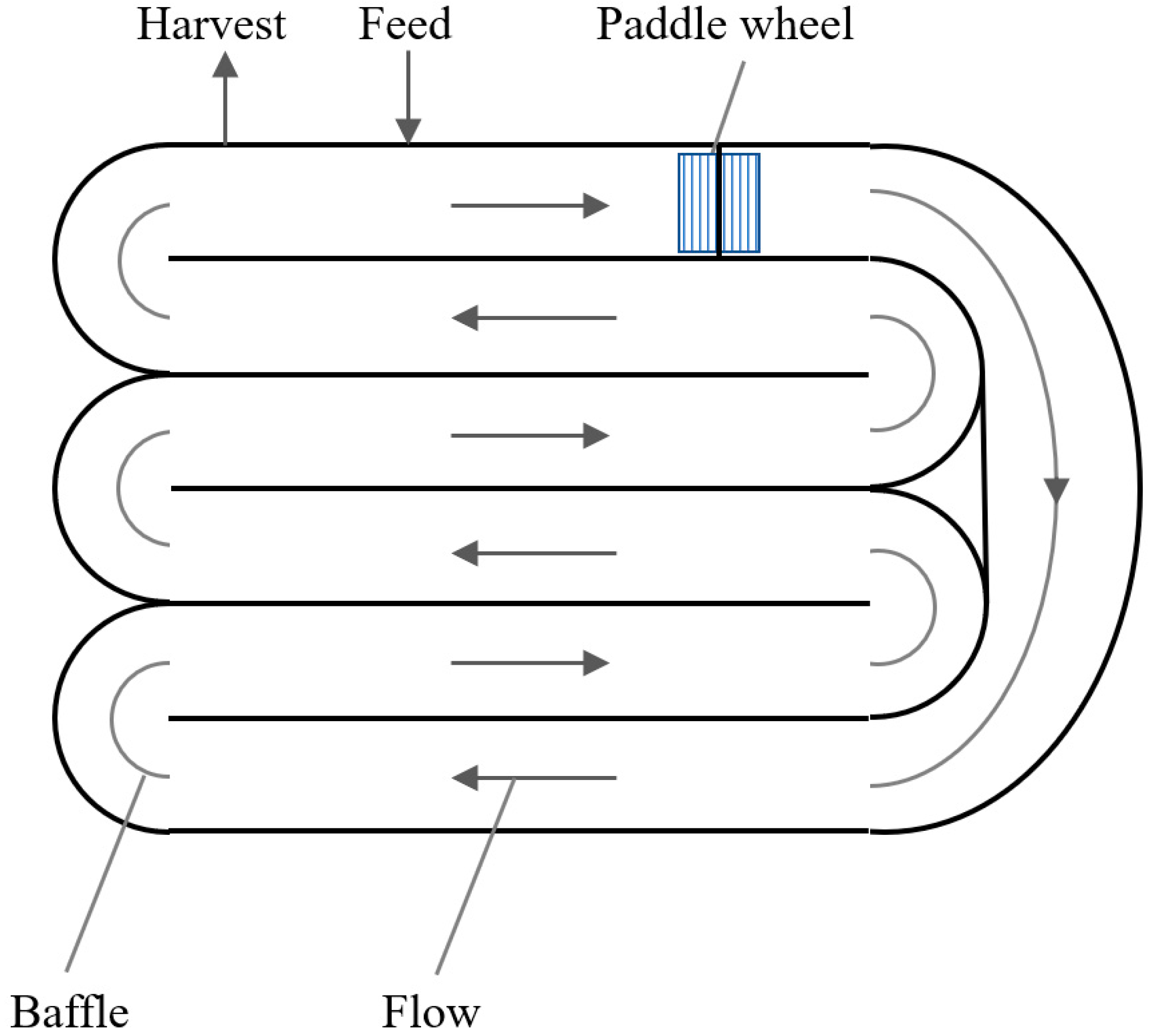
Figure iii. Culture of Arthrospira platensis in a bubble column type bioreactor. Slight foam formation and cell growth on the vessel walls were experienced (photo: Thousand. Murkovic).
Figure 3. Civilisation of Arthrospira platensis in a chimera column blazon bioreactor. Slight foam germination and jail cell growth on the vessel walls were experienced (photo: Chiliad. Murkovic).

Table one. Summary of microalgae cultivation strategies with regard to the main characteristics (STR PBR: stirred tank reactor photobioreactor).
Table 1. Summary of microalgae tillage strategies with regard to the primary characteristics (STR PBR: stirred tank reactor photobioreactor).
| Parameter | Open Ponds | Raceway Ponds | Bubble Columns | Tube Reactors | STR PBR |
|---|---|---|---|---|---|
| Mixing | very low | low | medium | medium | high |
| Illumination | very low | low | medium | loftier | medium |
| Sterility | no | low | possible | possible | yes |
| Upper-case letter and operational cost | very low | very low | medium | medium | high |
Table 2. Macronutrient composition of commercially important microalgae (extracted from [1,17,42,43].
Table ii. Macronutrient limerick of commercially important microalgae (extracted from [1,17,42,43].
| Species | Protein (wt%) | Carbohydrate (wt%) | Lipid (wt%) |
|---|---|---|---|
| Nannochloropsis sp. | 29–32 | nine–36 | xv–xviii |
| Nannochloropsis oceanica | 29 | 32–39 | 19–24 |
| Botryococcus braunii | 70 | – | – |
| Arthrospira platensis | 53–70 | 12–24 | 6–20 |
| Chlorella vulgaris | 49–55 | 7–42 | iii–36 |
| Haematococcus pluvialis | 48 | 27 | 15 |
| Isochrysis galbana | 27 | 17 | 17 |
| Dunaliella salina | 57 | 32 | 6 |
| Scenedesmus obliquus | 50–56 | ten–17 | 12–14 |
| Porphyridium cruentum | 28–39 | 40–57 | 9–fourteen |
Table 3. Polyunsaturated fatty acids in microalgae (extracted from [17,43]; given in % of total fat acids).
Table iii. Polyunsaturated fatty acids in microalgae (extracted from [17,43]; given in % of total fatty acids).
| Species | LA | ALA | GLA | STA | AA | EPA | DPA | DHA |
|---|---|---|---|---|---|---|---|---|
| Chlorella vulgaris | 3–10 | 2–21 | 15–24 | Traces | Traces | iii | 3 | 21 |
| Arthrospira platensis | x–21 | 1 | 1–25 | 1 | 0.3–0.iv | ii–3 | - | three |
| Isochrysis galbana | ane | 0.v | 0.5 | 1 | 1 | two | Traces | 19 |
| Scenedesmus obliquus | 1–2 | iv–22 | 0.1–4 | one–3 | 0.1–0.2 | - | - | - |
| Haematococcus pluvialis | four | 15 | 0.ane | - | - | - | - | - |
| Porphyridium cruentum | 0.4–25 | - | - | - | 1–35 | one–27 | - | half dozen.1 |
| Nannochloropsis oceanica | 0.vi–0.8 | - | - | - | 1.4–1.9 | 8.4–11 | - | - |
| Publisher'southward Notation: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This commodity is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Source: https://www.mdpi.com/2304-8158/10/7/1626/htm
0 Response to "What Is the Best Algae Strain to Eat"
Post a Comment